Subarea 1: Stem Cell Aging
The individual research groups within Subarea 1 investigate the causes and consequences of stem cell aging. The research work spans from basic model organisms over genetic mouse models up to humanized mouse models engrafted with human stem cells.
According to the FLI, with the closure of two groups since 2016 the representation of invertebrate models of stem cell research was reduced in Subarea 1. The institute presumes that the recruitment of new groups should fill this gap.
The research is defined by four focus areas:
- Cell-intrinsic mechanisms limiting the function of aging stem and progenitor cells,
- Aging-associated alterations of stem cell niches and the systemic environment,
- Mechanisms of clonal selection and epigenetic drifts in stem cell aging, and
- Microbiota- and metabolism-induced impairments in stem cell function during aging (in context of the new focus area Microbiota and Aging currently being built up within Subarea 2).
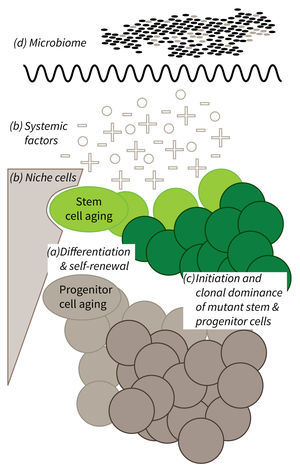
Research focus of Subarea 1.
a) It is currently not well understood what mechanisms impair cellular functions in aging. b) The relative contribution of niche cells and systemic acting factors on stem cell aging have yet to be determined in different tissues. c) Clonal expansion of mutant cells associates with disease development in aging humans. Mechanistically, the process remains poorly understood. Changes in color intensity depict clonal dominance originating from stem (green) or progenitor cells (gray). d) Emerging evidences indicate that aging associated alter ations in microbiota influence stem cell function and vice versa.
Publications
(since 2016)
2023
- Immunoproteasome function maintains oncogenic gene expression in KMT2A-complex driven leukemia.
Tubío-Santamaría N, Jayavelu AK, Schnoeder TM, Eifert T, Hsu CJ, Perner F, Zhang Q, Wenge DV, Hansen FM, Kirkpatrick JM, Jyotsana N, Lane SW, von Eyss B, Deshpande AJ, Kühn MWM, Schwaller J, Cammann C, Seifert U, Ebstein F, Krüger E, Hochhaus A, Heuser M, Ori A, Mann M, Armstrong SA, Heidel FH
Mol Cancer 2023, 22(1), 196 - Resident and recruited macrophages differentially contribute to cardiac healing after myocardial ischemia
Weinberger T, Messerer D, Joppich M, Fischer M, Garcia C, Kumaraswami K, Wimmler V, Ablinger S, Räuber S, Fang J, Liu L, Han Liu W, Winterhalter J, Lichti J, Tomas L, Esfandyari D, Percin G, Martin Salamanca S, Hidalgo A, Waskow C, Engelhardt S, Todica A, Zimmer R, Pridans C, Gomez-Perdiguero E, Schulz C
bioRxiv 2023, 10.1101/2023.05.08.539799
2022
- Vitamin A metabolism in niche cells activates retinoic acid signaling and impairs stem cell function and skeletal muscle maintenance in aging mice
Becker F
Dissertation 2022, Jena - Impaired formation of neutrophil extracellular traps (NETs) in patients with myelodysplastic syndrome.
Brings C, Fröbel J, Cadeddu RP, Germing U, Haas R, Gattermann N
BLOOD ADV 2022, 6(1), 129-37 - Combined Activity of the Redox-Modulating Compound Setanaxib (GKT137831) with Cytotoxic Agents in the Killing of Acute Myeloid Leukemia Cells.
Demircan MB, Mgbecheta PC, Kresinsky A, Schnoeder TM, Schröder K, Heidel FH, Böhmer FD
Antioxidants (Basel) 2022, 11(3) - Context-specific effects of NOX4 inactivation in acute myeloid leukemia (AML).
Demircan MB, Schnoeder TM, Mgbecheta PC, Schröder K, Böhmer FD, Heidel FH
J Cancer Res Clin Oncol 2022, 148(8), 1983-90 - Histone demethylase KDM4C is a functional dependency in JAK2-mutated neoplasms.
Ernst P, Schnöder TM, Huber N, Perner F, Jayavelu AK, Eifert T, Hsu CJ, Tubío-Santamaría N, Crodel CC, Ungelenk M, Hübner CA, Clement JH, Hochhaus A, Heidel FH
Leukemia 2022, 36(7), 1843-9 - Muscle stem cell maintenance and regeneration of skeletal muscle require functional motor innervation
Henze H
Dissertation 2022, Jena, Germany - Taz protects hematopoietic stem cells from an aging-dependent decrease in PU.1 activity.
Kim* KM, Mura-Meszaros* A, Tollot* M, Krishnan MS, Gründl M, Neubert L, Groth M, Rodriguez-Fraticelli A, Svendsen AF, Campaner S, Andreas N, Kamradt T, Hoffmann S, Camargo FD, Heidel FH, Bystrykh LV, de Haan G, von Eyss B
Nat Commun 2022, 13(1), 5187 * equal contribution - Meeting Report: Aging Research and Drug Discovery
Meron E, Thaysen M, Angeli S, Antebi A, Barzilai N, Baur JA, Bekker-Jensen S, Birkisdottir M, Bischof E, Bruening J, Brunet A, Buchwalter A, Cabreiro F, Cai S, Chen BH, Ermolaeva M, Ewald Collin Y, Ferrucci L, Florian MC, Fortney K, Freund A, Georgievskaya A, Gladyshev VN, Glass D, Golato T, Gorbunova V, Hoejimakers J, Houtkooper RH, Jager S, Jaksch F, Janssens G, Borch Jensen M, Kaeberlein M, Karsenty G, de Keizer P, Kennedy B, Kirkland JL, Kjaer M, Kroemer G, Lee KF, Lemaitre JM, Liaskos D, Longo VD, Lu YX, MacArthur MR, Maier AB, Manakanatas C, Mitchell SJ, Moskalev A, Niedernhofer L, Ozerov I, Partridge L, Passegué E, Petr MA, Peyer J, Radenkovic D, Rando TA, Rattan S, Riedel CG, Rudolph L, Ai R, Serrano M, Schumacher B, Sinclair DA, Smith R, Suh Y, Taub P, Trapp A, Trendelenburg AU, Valenzano DR, Verburgh K, Verdin E, Vijg J, Westendorp RGJ, Zonari A, Bakula D, Zhavoronkov A, Scheibye-Knudsen M
Aging (Albany NY) 2022, 14(2), 530–543