Subarea 1: Stem Cell Aging
The individual research groups within Subarea 1 investigate the causes and consequences of stem cell aging. The research work spans from basic model organisms over genetic mouse models up to humanized mouse models engrafted with human stem cells.
According to the FLI, with the closure of two groups since 2016 the representation of invertebrate models of stem cell research was reduced in Subarea 1. The institute presumes that the recruitment of new groups should fill this gap.
The research is defined by four focus areas:
- Cell-intrinsic mechanisms limiting the function of aging stem and progenitor cells,
- Aging-associated alterations of stem cell niches and the systemic environment,
- Mechanisms of clonal selection and epigenetic drifts in stem cell aging, and
- Microbiota- and metabolism-induced impairments in stem cell function during aging (in context of the new focus area Microbiota and Aging currently being built up within Subarea 2).
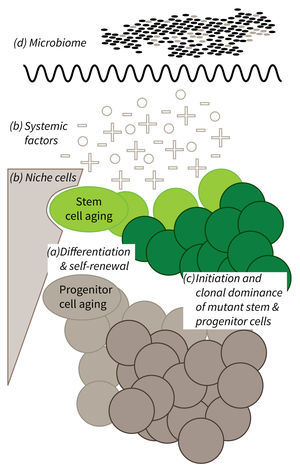
Research focus of Subarea 1.
a) It is currently not well understood what mechanisms impair cellular functions in aging. b) The relative contribution of niche cells and systemic acting factors on stem cell aging have yet to be determined in different tissues. c) Clonal expansion of mutant cells associates with disease development in aging humans. Mechanistically, the process remains poorly understood. Changes in color intensity depict clonal dominance originating from stem (green) or progenitor cells (gray). d) Emerging evidences indicate that aging associated alter ations in microbiota influence stem cell function and vice versa.
Publications
(since 2016)
2016
- Neuron-Specific Deletion of the Nf2 Tumor Suppressor Impairs Functional Nerve Regeneration.
Schulz A, Büttner R, Toledo A, Baader SL, von Maltzahn J, Irintchev A, Bauer R, Morrison H
PLoS One 2016, 11(7), e0159718 - Epigenetic stress responses induce muscle stem-cell ageing by Hoxa9 developmental signals.
Schwörer S, Becker F, Feller C, Baig AH, Köber U, Henze H, Kraus JM, Xin B, Lechel A, Lipka DB, Varghese CS, Schmidt M, Rohs R, Aebersold R, Medina KL, Kestler HA, Neri F, von Maltzahn** J, Tümpel** S, Rudolph** KL
Nature 2016, 540(7633), 428-32 ** co-corresponding authors - Dietary restriction improves repopulation but impairs lymphoid differentiation capacity of hematopoietic stem cells in early aging.
Tang D, Tao S, Chen Z, Koliesnik IO, Calmes PG, Hoerr V, Han B, Gebert N, Zörnig M, Löffler B, Morita** Y, Rudolph** KL
J Exp Med 2016, 213(4), 535-53 ** co-corresponding authors - Characterization of SKAP/Kinastrin isoforms - The N-terminus defines tissue specificity and Pontin binding.
Vranesic AC, Reiche J, Hoischen C, Wohlmann A, Bratsch J, Friedrich K, Günes B, Cappallo-Obermann H, Kirchhoff C, Diekmann S, Günes C, Huber O
Hum Mol Genet 2016, 25(13), 2838-52 - SIRT6 Controls Hematopoietic Stem Cell Homeostasis through Epigenetic Regulation of Wnt Signaling.
Wang H, Diao D, Shi Z, Zhu X, Gao Y, Gao S, Liu X, Wu Y, Rudolph KL, Liu G, Li T, Ju Z
Cell Stem Cell 2016, 18(4), 495-507 - Per2 induction limits lymphoid-biased haematopoietic stem cells and lymphopoiesis in the context of DNA damage and ageing.
Wang J, Morita Y, Han B, Niemann S, Löffler B, Rudolph KL
Nat Cell Biol 2016, 18(5), 480-90 recommended by "Faculty of 1000" - Prime - Classical and alternative NF-κB signaling cooperate in regulating adipocyte differentiation and function.
Weidemann A, Lovas A, Rauch A, Andreas N, von Maltzahn J, Riemann M, Weih F
Int J Obes Relat Metab Disord 2016, 40(3), 452-9