Animal experiments at the FLI
At the Leibniz Institute on Aging - Fritz Lipmann Institute (FLI), scientists are investigating the biological basis of aging. Since aging is a process that affects the entire organism, many scientific questions can only be investigated using animal models. This makes the FLI one of that use animal experiments in their research.
As with all experiments conducted in the FLI laboratories,experiments on animals have the aim of better understanding the biological processes that change with aging. These include changes in stem cell functionality, tissue homeostasis, regenerative capacity and the microbiome. Findings from such studies can lead to the development of new therapeutic options to improve human health in old age.
To gain insights into these biological processes, scientists at the FLI use and two fish species, the and , as model organisms. When researchers plan scientific experiments, they always first consider whether experiments with vertebrates are necessary or whether other methods are sufficient to answer the scientific question.
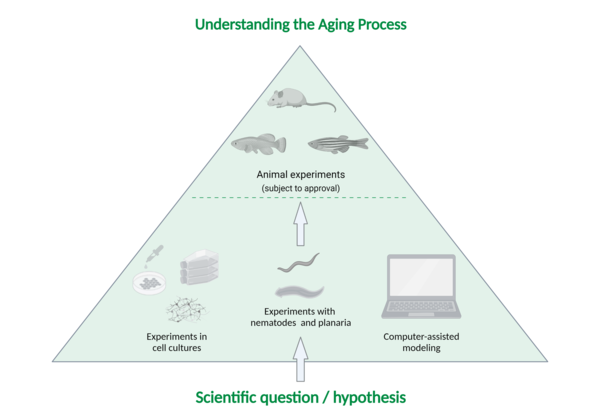
They proceed according to the following system (see image below):
First step: Generating a hypothesis/scientific question
Second step: Conducting scientific experiments using cell cultures (or other experimental methods)
Only if a hypothesis cannot be tested by experiments with cells or with the help of other methods such as computer simulation or studies on worms do researchers consider animal experiments with vertebrates.
The performance of these animal experiments is strictly regulated by the Animal Welfare Act and requires a multi-stage approval process. They are planned and carried out exclusively by persons who have the legally required knowledge and skills. These are primarily bioscientists and veterinarians, but also animal caretakers and laboratory technicians who have completed courses in laboratory animal science to obtain an officially recognized certificate of competence.
The scientists and their colleagues involved in these animal experiments are aware of their responsibility towards animals at all times. In order to avoid animal experiments (Replace), to minimize the number of animals in experiments (Reduce) and to improve animal welfare (Refine), they apply the 3R principle and thus commit themselves to always safeguarding animal welfare, even beyond the legal requirements.
In addition, animal husbandry and animal welfare at the FLI are subject to an internal Compliance Management System (CMS), in which a compliance staff and experts ensure that all scientific procedures are carried out in accordance with the regulations.
Animal Welfare Act
Germany has had an (in German only) since 1972. This act protects all animals – including laboratory animals – and was revised in 2006, 2013 and 2021 with the aim of protecting animal welfare. Section 1 states: “The purpose of this law is to protect the life and well-being of animals based on human responsibility for them as fellow creatures. No one may inflict pain, suffering or harm on an animal without reasonable cause.” Thus, the Animal Welfare Act permits animal experimentation – as long as there are “reasonable grounds.” Such reasonable grounds, valid prior to the Act, are listed in Section 7a. These include basic research or other research aimed at the “prevention, detection or treatment of disease, suffering, physical injury or physical discomfort in humans or animals.”
Directive 2010/63/EU and Animal Welfare Regulation Governing Experimental Animals
As far as possible, animals should be subject to the same requirements for protection in all member states of the European Union. This also makes the scientific results of animal experiments more comparable. The has since become national law in all EU countries. Thus in Germany the Animal Welfare Act was amended in 2013 and animal welfare law was further refined through the Animal Welfare Regulation Governing Experimental Animals (Tierschutz-Versuchstierverordnung). In 2021, the law was further amended and updated.
The 3R principle is the fundamental ethical principle of laboratory animal science. Its aim is to reduce the frequency of animal experiments to what is absolutely necessary and to minimize the suffering of each individual animal as much as possible. This principle was anchored in the European Union Directive 2010/63/EU and is thus legally binding for all EU states (see above). The requirements of this EU directive were incorporated into German national law in 2013.
The 3R principle was established in 1959 by William Russel and Rex Burch in their book .
What do the three Rs stand for?
Replacement: As far as possible, animal experiments should be replaced with alternative methods.
Reduction: Animal testing and the number of animals used should be reduced to the absolute minimum necessary.
Refinement: The stress on the animals during the experiment must be kept as low as possible and their housing conditions constantly improved.
How is the 3R principle implemented at the FLI?
Replacement – Avoiding animal experimentation
Whenever possible, scientific questions are first tested in cell culture (in vitro) before an animal experiment is planned and carried out (in vivo). Another approach is virtual experiments using computer models (in silico). To enable this type of experimentation, the Department of Bioinformatics at FLI has been further expanded. In addition, there is the possibility at the FLI to avoid animal experiments on vertebrates such as mice and fish by performing studies on invertebrate species such as nematodes or on fish eggs and larvae.
Reduction – Reducing the number of animals involved in experiments
In order to obtain meaningful results, a thorough experimental design is essential before the start of the experiment. In addition to advice from animal welfare officers, scientists at the FLI are required to consult biostatisticians. With the help of statistical methods, group sizes and the number of animals involved can be calculated in such a way that they produce meaningful results with the fewest subjects. Such careful experimental design helps to reduce the number of animals per experiment and, above all, to avoid repetition of animal experiments. In addition, a database has been programmed at the FLI that can be used to transfer animals between scientists at the FLI. For example, several scientific questions can be answered with the help of a single laboratory animal. This helps to keep the number of animals used as low as possible.
Refinement – Improvement of living conditions and treatment
Close cooperation between animal caretakers, veterinarians, researchers and animal welfare officers ensures that animals are optimally monitored and cared for in animal husbandry as well as in animal experiments. New approaches are regularly discussed and tested in the . Thus, animal welfare at the FLI is being continuously improved.
There are different types of experiments in which the animals’ well-being is affected to a greater or lesser extent. While drawing blood causes only minor pain, an operation in which, for example, nerves are cut to study the effects of muscle atrophy can cause more severe and longer-lasting pain or impairment.
Anyone wishing to carry out an animal experiment must, in addition to the legally required training and approval, assess and document – before, during and after the experiment – how the stress on the animals is to be classified. “Stress” refers to the pain, suffering, distress and lasting harm that an animal experiences, and the classification of procedures considers the measures with which this can be minimized. The European Union Directive 2010/63/EU “On the protection of animals used for scientific purposes” distinguishes between four categories of procedures:
- Mild: Animal procedures in which animals are subjected to brief and minor pain, suffering or harm, such as a single blood withdrawal or injection. These are procedures that are also routine in a veterinary practice.
- Moderate: Animal experiments in which animals are subjected to short periods of moderate pain, suffering or harm, or longer periods of minor pain, for example, during surgical procedures under general anesthesia followed by drug treatment for pain.
- Severe: Animal experiments that cause severe pain, suffering or harm to an animal or prolonged moderate pain, suffering or harm that could result in the death of the animal.
- Non-recovery: Animal experiments in which animals that have not previously undergone any procedures are killed for scientific purposes – for example, to remove individual organs; or experiments in which animals are placed under general anesthesia (to undergo minor surgery), and are then euthanized while still unconscious.
The regularly surveys how many laboratory animals are exposed to which degree of stress.
In 2020, just under 1.9 million animals were used for animal experiments in Germany. Added to this is the number of vertebrate animals killed for scientific purposes, for example to remove organs or tissue for examination. If animals are killed without having first undergone experimental procedures, this is not an animal experiment in the sense of the law, but these figures are nevertheless included in the annual laboratory animal report. The figures are regularly published on the homepage of the .
Like other EU countries, Germany is also legally obliged to report animal experiments. The exact procedure is regulated in the (in German only). The report should record the type, origin and number of all vertebrate or cephalopod animals used, as well as the purpose, nature and severity of the experiments.
Before an animal experiment can be conducted in Germany, it must be approved by the competent authority. For the FLI, the responsible body is the Thuringian State Office for Consumer Protection (TLV), a higher authority of the State of Thuringia.
The application approval process begins when the scientist fills out an animal experiment application. This application must explain comprehensively and in detail why the animal experiment is indispensable to answer a specific scientific question and why animals must be used for this purpose. This means that the scientist must always first determine whether another method could be used instead of an animal experiment to answer the question. In addition, applicants must show that they have extensively researched whether or not the question has already been investigated elsewhere in the world. Furthermore, it is required that the animal experiment takes place under consideration of the 3R principle (see above).
In the next step, the animal experiment application is reviewed by the animal welfare officer of the FLI according to the aspects mentioned above. This person advises the applicant and makes suggestions with regard to the implementation of animal welfare measures. Together with the animal experiment application, the animal welfare officer then sends his or her independent assessment of the planned animal experiment to the competent authority. The authority examines the application and is supported in an advisory capacity by an animal protection commission (the so-called §15 commission). This commission is made up of experts such as scientists, ethicists, statisticians and non-scientific representatives of animal protection organizations, who also evaluate the application according to the above-mentioned criteria. Only if an application fulfills all criteria relevant to animal protection law will it be approved.
Once an application has been approved, the animal experiment may be carried out. Further controls take place during the conducting of the animal experiment and with regard to its documentation – internally by the animal welfare officer and externally by the veterinary authority. The official veterinary controls are partly unannounced on-site inspections. In addition, interim and final reports are requested from the scientists, documenting data and facts about the course of the animal experiment. If deviations are found during the inspections by animal welfare officers or the veterinary office, the animal experiment can be stopped immediately.