FLI Compliance Management
The FLI experienced two profound crises in 2016 and 2017. The first was related to rule violations in animal husbandry, the second to rule violations in scientific publication practices.
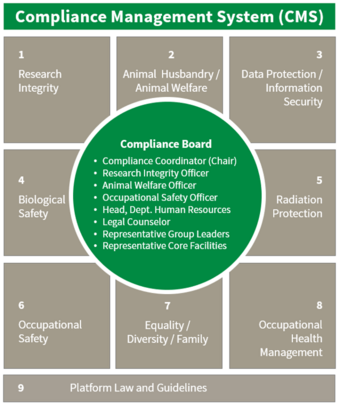
Compliance Management System (CMS)
As part of the crisis management, a Compliance Management System (CMS) was established at the FLI in October 2018. It aims to ensure compliance with legal and operational guidelines by documenting processes and by communicating, in a transparent manner, any issues that arise.
Focus on science-related processes
The focus of the work of the CMS Board and the compliance experts from eight working areas – including research, animal husbandry, occupational safety, data protection, health management, and equality – is the review of science-related processes.
Compliance Reports
The CMS Board submits a confidential compliance report to the FLI Board of Directors every year.
Good Scientific Practice
Part of compliance management at the FLI is Good Scientific Practice (GSP). The principles of GSP, such as verifiability and transparency, are intended to ensure the quality and thus integrity and trustworthiness of scientific work.
The GWP standards of the FLI are regularly reviewed and further developed. This includes, for example, sustainable management of research data and a data steward who promotes and guides such activities at the FLI. More information can be found .
Contact
Jens Bösger
Health & Safety Officer
+49 3641 65-6072
Jens.Boesger@~@leibniz-fli.de