Correia-Melo Research Group
Host-Microbiome Metabolism in Aging
Cells not only sense and take up nutrients from their surrounding environment, they also release a broad range of metabolites produced as a result of their biosynthetic activity. This metabolite release from cells, which is essential for maintaining metabolic balance within the cell (homeostasis), creates specific metabolic microenvironments with important physiological consequences, particularly when it comes to regulating survival and response to therapies.
The network of chemical reactions within the cell, also termed the intracellular metabolic network, has been extensively characterized, yet our understanding remains limited with regard to the metabolic microenvironment surrounding the cell as a modulator of cellular and tissue function, as well as its impact on disease initiation, progression, and therapy response.
Looking for answers
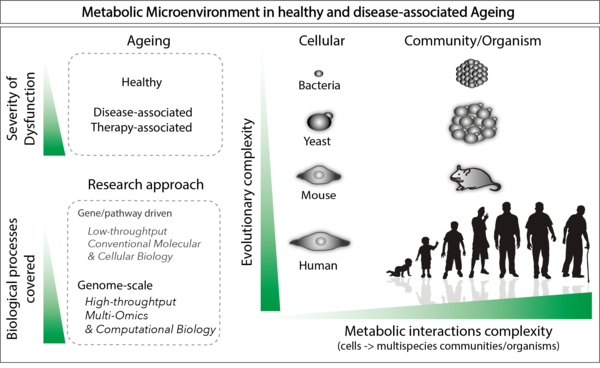
By examining the metabolic microenvironments that emerge from cell-cell metabolite exchange interactions in both healthy and disease-associated aging, we hope to answer outstanding questions in the field:
- How do metabolites secreted by cells alter the physiology of neighboring cells?
- How does this impact aging, disease initiation, and progression?
- How do host and microbiome cells interact metabolically?
- How do clinically used therapeutics affect the metabolic environment?
- Can we target metabolic pathways or use metabolic adjuvants to aid therapy efficiency?
State-of-the-art analyses
Our lab aims at building a systems-scale mechanistic understanding of the metabolic microenvironments that modulate health, disease, and response to therapy, with a special focus on aging. For this we use high-throughput mass spectrometry (MS)-based metabolomics and proteomics, coupled with low-throughput molecular biology approaches, in both mammalian and microbial systems.
The lab focuses on three major areas:
- Host-microbiome metabolic crosstalk during aging.
- Metabolic microenvironment as a driver of disease initiation and progression.
- Metabolic microenvironment generated by therapy-induced senescent cells (TIS cells) as a factor in disease persistence.
Discoveries at all three levels have the potential to translate into interventions that support better health through innovative metabolism-targeting or metabolism-aided therapies.
Contact

Clara Correia-Melo
Group Leader
+49 3641 65-6323
clara.correia-melo@~@leibniz-fli.de
Ramona Schwarz
Assistance
+49 3641 65-6385
ramona.schwarz@~@leibniz-fli.de