Subarea 2: Regeneration and Homeostasis of Organs in Aging
The main goal of Subarea 2 is to identify cellular and molecular pathways used to ensure effective organ maintenance and repair, and to unravel the mechanisms of their deterioration during aging. While stem cells are important for organ homeostasis, this Subarea does not per se directly addresses stem cell aging but rather focusses on the following focus areas:
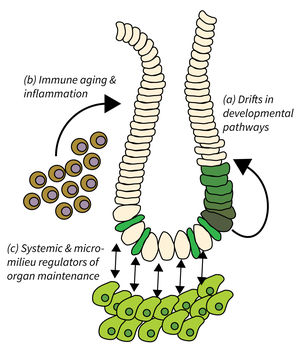
- Drifts in developmental pathways limiting organ maintenance in aging,
- Immune aging and inflammation, and
- Systemic and micro-milieu regulators of organ maintenance, regeneration, and disease development.
Research focus of Subarea 2
Organ maintenance is regulated by local and systemic factors, which are subject to aging-associated changes. Research of Subarea 2 focuses on the following research areas: a) Genetic and epigenetic modulation of developmental pathways has been shown to contribute to progressive aging and disease. It is critical to delineate mechanisms and consequences of aging-associated drifts to better understand organ maintenance during aging. b) Immunoaging and chronic inflammation elicits negative effects through reduced immune surveillance and aberrant organ repair and maintenance; all of which contributes to the evolution of organ pathologies and diseases during organismal aging. c) Furthermore, aging-associated alterations in systemic and extracellular factors derived from metabolic changes, microbiota alterations, chronic inflammation, senescent, or damaged cells might impinge on disease development and tumor initiation.
Publications
(since 2016)
2016
- Multifocal Nerve Lesions and LZTR1 Germline Mutations in Segmental Schwannomatosis.
Farschtschi S, Mautner VF, Pham M, Nguyen R, Kehrer-Sawatzki H, Hutter S, Friedrich RE, Schulz A, Morrison H, Jones DTW, Bendszus M, Bäumer P
Ann Neurol 2016, 80(4), 625-8 - The putative oncogene CPI-17 is up-regulated in schwannoma.
Hagel C, Dornblut C, Schulz A, Wiehl U, Friedrich RE, Huckhagel T, Mautner VF, Morrison H
Neuropathol Appl Neurobiol 2016, 42(7), 664-8 - Feedback activation of neurofibromin terminates growth factor-induced Ras activation.
Hennig A, Markwart R, Wolff K, Schubert K, Cui Y, Prior IA, Esparza-Franco MA, Ladds G, Rubio I
Cell Commun Signal 2016, 14(1), 5 - Shwachman-Bodian-Diamond syndrome (SBDS) protein deficiency impairs translation re-initiation from C/EBPα and C/EBPβ mRNAs.
In K, Zaini MA, Müller C, Warren AJ, von Lindern M, Calkhoven CF
Nucleic Acids Res 2016, 44(9), 4134-46 - Role of the alternative NF-kB signaling in T cell development and functions
Koliesnik I
Dissertation 2016, Jena, Germany - High-Content Microscopy Analysis of Subcellular Structures: Assay Development and Application to Focal Adhesion Quantification.
Kroll* T, Schmidt* D, Schwanitz G, Ahmad M, Hamann J, Schlosser C, Lin YC, Böhm KJ, Tuckermann J, Ploubidou A
Curr Protoc Cytom 2016, 77, 12.43.1-12.43.44 * equal contribution - Menin is a tumor suppressor in bone : a novel benign jaw tumor mouse model
Lee S
Dissertation 2016, Ulm, Germany - Impaired Planar Germ Cell Division in the Testis, Caused by Dissociation of RHAMM from the Spindle, Results in Hypofertility and Seminoma.
Li H, Frappart* L, Moll* J, Winkler* A, Kroll T, Hamann J, Kufferath I, Groth M, Taudien S, Schütte M, Yaspo ML, Heuer H, Lange BMH, Platzer M, Zatloukal K, Herrlich P, Ploubidou A
Cancer Res 2016, 76(21), 6382-95 * equal contribution - Dicer ablation in osteoblasts by Runx2 driven cre-loxP recombination affects bone integrity, but not glucocorticoid-induced suppression of bone formation.
Liu P, Baumgart M, Groth M, Wittmann J, Jäck HM, Platzer M, Tuckermann* JP, Baschant* U
Sci Rep 2016, 6, 32112 * equal contribution - Different promoter affinities account for specificity in MYC-dependent gene regulation.
Lorenzin F, Benary U, Baluapuri A, Walz S, Jung LA, von Eyss B, Kisker C, Wolf J, Eilers M, Wolf E
Elife 2016, 5. pii: e15161, doi: 10.7554/eLife.15161.