Subarea 2: Regeneration and Homeostasis of Organs in Aging
The main goal of Subarea 2 is to identify cellular and molecular pathways used to ensure effective organ maintenance and repair, and to unravel the mechanisms of their deterioration during aging. While stem cells are important for organ homeostasis, this Subarea does not per se directly addresses stem cell aging but rather focusses on the following focus areas:
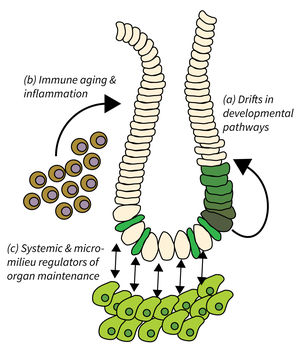
- Drifts in developmental pathways limiting organ maintenance in aging,
- Immune aging and inflammation, and
- Systemic and micro-milieu regulators of organ maintenance, regeneration, and disease development.
Research focus of Subarea 2
Organ maintenance is regulated by local and systemic factors, which are subject to aging-associated changes. Research of Subarea 2 focuses on the following research areas: a) Genetic and epigenetic modulation of developmental pathways has been shown to contribute to progressive aging and disease. It is critical to delineate mechanisms and consequences of aging-associated drifts to better understand organ maintenance during aging. b) Immunoaging and chronic inflammation elicits negative effects through reduced immune surveillance and aberrant organ repair and maintenance; all of which contributes to the evolution of organ pathologies and diseases during organismal aging. c) Furthermore, aging-associated alterations in systemic and extracellular factors derived from metabolic changes, microbiota alterations, chronic inflammation, senescent, or damaged cells might impinge on disease development and tumor initiation.
Publications
(since 2016)
2025
- Analysis of microRNA expression reveals convergent evolution of the molecular control of diapause in annual killifishes.
Barth* E, Baumgart* M, Dolfi* L, Cui R, Groth M, Ripa R, Savino A, Valenzano DR, Platzer M, Marz** M, Cellerino** A
Front Genet 2025, 16, 1583989 * equal contribution, ** co-corresponding authors - Recombinant Human Neuregulin1-β1 Significantly Reduces Schwannoma Growth in Mice.
Bischoff JP, Schulz A, Hagel C, Büttner R, Reuter M, Bessler WK, Clapp DW, Morrison H
Ann Neurol 2025 (epub ahead of print) - Age-dependent decline in sperm quality and function in a naturally short-lived vertebrate.
Cattelan** S, Valenzano** DR
BMC Ecol Evol 2025, 25(1), 2 ** co-corresponding authors - Altered translation elongation contributes to key hallmarks of aging in the killifish brain.
Di Fraia* D, Marino* A, Lee* JH, Kelmer Sacramento E, Baumgart M, Bagnoli S, Balla T, Schalk F, Kamrad S, Guan R, Caterino C, Giannuzzi C, Tomaz da Silva P, Sahu AK, Gut H, Siano G, Tiessen M, Terzibasi-Tozzini E, Fornasiero EF, Gagneur J, Englert C, Patil KR, Correia-Melo C, Nedialkova DD, Frydman** J, Cellerino** A, Ori** A
Science 2025, 389(6759), eadk3079 * equal contribution, ** co-senior authors - Microglia and CD8+ T cell activation precede neuronal loss in a murine model of spastic paraplegia 15.
Frolov A, Huang H, Schütz D, Köhne M, Blank-Stein N, Osei-Sarpong C, Büttner M, Elmzzahi T, Khundadze M, Zahid M, Reuter M, Becker M, De Domenico E, Bonaguro L, Kallies A, Morrison H, Hübner CA, Händler K, Stumm R, Mass E, Beyer MD
J Exp Med 2025, 222(7), e20232357 - Analysis of different strains of the turquoise killifish identify transcriptomic signatures associated with heritable lifespan differences.
Mazzetto M, Reichwald K, Koch P, Groth M, Cellerino A
J Gerontol A Biol Sci Med Sci 2025, 80(7), glae255 - Mechanistic causes of immune-senescence in the immune niche of killifish aging model
Morabito G
Dissertation 2025, Jena, Germany - Signatures of Nonlinear Aging: Molecular Stages of Life: Sudden Changes During Aging as Potential Biomarkers for an Age Classification System.
Olecka** M, Morrison H, Hoffmann** S
Bioessays 2025, 47(5), e202400222 ** co-corresponding authors - Emerging roles of transfer RNA fragments in the CNS.
Winek** K, Soreq** H
Brain 2025, 148(8), 2631-45 ** co-corresponding authors