Subarea 4: Cell Dynamics and Molecular Damages in Aging
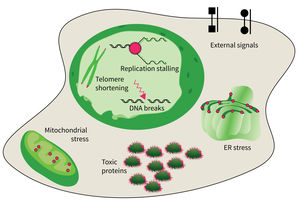
The research focus of Subarea 4 is on studying damages of macromolecules (proteins, nucleic acids) and determining the structure-function relationship of biomolecules relevant to damage and damage repair processes and responses to molecular damage that might lead to aging and aging-associated pathologies.
The studies are focused on the following research areas: DNA replication, DNA damage responses (DDR), stress responses, metabolic stresses, protein trafficking and protein damages.
The research is defined by four focus areas:
- DNA damage response in tissue homeostasis and neuropathies,
- Quality control in the endoplasmic reticulum for secretory pathway in aging processes,
- Intrinsic and extrinsic factors implicated in cellular decline during aging, and
- DNA replication and genomic integrity preventing premature aging and diseases.
Research focus of Subarea 4.
The accumulation of damaged macromolecules or subcellular organelles is associated with dysfunction of a cell, which contributes to tissue & organ failure. DNA damage, genomic instability, protein misfolding or defects in toxic protein degradation can compromise cell functionality. Alterations of mitochondrial DNA and protein complexes affect cellular metabolism, which will have a general impact on cell integrity.
Publications
(since 2016)
2018
- LSD1 (KDM1A)-independent effects of the LSD1 inhibitor SP2509 in cancer cells.
Sonnemann J, Zimmermann M, Marx C, Ebert F, Becker S, Lauterjung ML, Beck JF
Br J Haematol 2018, 183(4), 494-7
2017
- Toxic effects of zinc ions on kinesin - Potential molecular cause of impaired intracellular transport.
Böhm KJ
Toxicol Lett 2017, 268, 58-62 - Myofibroblasts have an impact on expression, dimerization and signaling of different ErbB receptors in OSCC cells.
Büttner R, Berndt A, Valkova C, Richter P, Korn A, Kosan C, Liebmann C
J Recept Signal Transduct Res 2017, 37(1), 25-37 - Structural Studies Revealed Active Site Distortions of Human Furin by a Small Molecule Inhibitor.
Dahms SO, Jiao** GS, Than** ME
ACS Chem Biol 2017, 12(5), 1211-6 ** co-corresponding authors - Neuroendocrine functions of the anti-aging factor Klotho
Doycheva D
Dissertation 2017, Jena, Germany - Raman and Infrared Spectroscopy Distinguishing Replicative Senescent from Proliferating Primary Human Fibroblast Cells by Detecting Spectral Differences Mainly Due to Biomolecular Alterations.
Eberhardt K, Beleites C, Marthandan S, Matthäus C, Diekmann S, Popp J
Anal Chem 2017, 89(5), 2937-47 - Raman and infrared spectroscopy differentiate senescent from proliferating cells in a human dermal fibroblast 3D skin model.
Eberhardt K, Matthäus C, Winter D, Wiegand C, Hipler UC, Diekmann S, Popp J
Analyst 2017, 142(23), 4405-14 - Characterization of the interaction between the small RNA-encoded peptide SR1P and GapA from Bacillus subtilis.
Gimpel M, Maiwald C, Wiedemann C, Görlach M, Brantl S
Microbiology 2017, 163(8), 1248-59 Editors' Choice - XRCC1 mutation is associated with PARP1 hyperactivation and cerebellar ataxia.
Hoch NC, Hanzlikova H, Rulten SL, Tétreault M, Komulainen E, Ju L, Hornyak P, Zeng Z, Gittens W, Rey SA, Staras K, Mancini GMS, McKinnon PJ, Wang ZQ, Wagner JD, Care4Rare Canada Consortium, Yoon G, Caldecott KW
Nature 2017, 541(7635), 87-91 - PARP1 controls KLF4-mediated telomerase expression in stem cells and cancer cells.
Hsieh MH, Chen YT, Chen YT, Lee YH, Lu J, Chien CL, Chen HF, Ho HN, Yu CJ, Wang ZQ, Teng SC
Nucleic Acids Res 2017, 45(18), 10492-503