Subarea 4: Cell Dynamics and Molecular Damages in Aging
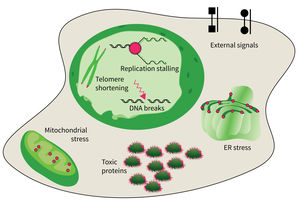
The research focus of Subarea 4 is on studying damages of macromolecules (proteins, nucleic acids) and determining the structure-function relationship of biomolecules relevant to damage and damage repair processes and responses to molecular damage that might lead to aging and aging-associated pathologies.
The studies are focused on the following research areas: DNA replication, DNA damage responses (DDR), stress responses, metabolic stresses, protein trafficking and protein damages.
The research is defined by four focus areas:
- DNA damage response in tissue homeostasis and neuropathies,
- Quality control in the endoplasmic reticulum for secretory pathway in aging processes,
- Intrinsic and extrinsic factors implicated in cellular decline during aging, and
- DNA replication and genomic integrity preventing premature aging and diseases.
Research focus of Subarea 4.
The accumulation of damaged macromolecules or subcellular organelles is associated with dysfunction of a cell, which contributes to tissue & organ failure. DNA damage, genomic instability, protein misfolding or defects in toxic protein degradation can compromise cell functionality. Alterations of mitochondrial DNA and protein complexes affect cellular metabolism, which will have a general impact on cell integrity.
Publications
(since 2016)
2025
- A fluorescent folding reporter uncovers myosin misfolding as a driver of Hypertrophic Cardiomyopathy
Arnese R, Julian Dexheimer P, Escobar-Doncel B, Krüger L, Adhikari G, Krpan N, Sehr D, Kley J, Deszcz L, Meinhart A, Kirstein J, Clausen T
bioRxiv 2025, https://doi.org/10.1101/2025.02. - The microcephaly-associated protein YIPF5 differentially regulates ER-export
Bruno F, Anitei M, Di Fraia D, Durso W, Dau T, Cirri E, Sannai M, Valkova C, Maldutyte J, A.Miller E, Rubio I, Garloff V, Kersten N, Farias G, Ori A, Mestres I, Calegari F, Kaether C
bioRxiv 2025, https://doi.org/10.1101/2025.06. - Translational Remodeling of the Synaptic Proteome During Aging.
Caterino C, Ugolini M, Durso W, Jevdokimenko K, Groth M, Riege K, Görlach M, Fornasiero E, Ori A, Hoffmann S, Cellerino A
Aging Cell 2025 (epub ahead of print) - Function of the anti-aging protein Klotho in the Choroid plexus
Fanaeikahrani Z
Dissertation 2025, Jena, Germany - Attenuated growth factor signaling during cell death initiation sensitizes membranes towards peroxidation.
Gollowitzer A, Pein H, Rao Z, Waltl L, Bereuter L, Loeser K, Meyer T, Jafari V, Witt F, Winkler R, Su F, Große S, Thürmer M, Grander J, Hotze M, Harder S, Espada L, Magnutzki A, Gstir R, Weinigel C, Rummler S, Bonn G, Pachmayr J, Ermolaeva M, Harayama T, Schlüter H, Kosan C, Heller R, Thedieck K, Schmitt M, Shimizu T, Popp J, Shindou H, Kwiatkowski M, Koeberle A
Nat Commun 2025, 16(1), 1774 - Exploring the effects of amyloid beta (Aß) and Tau aggregation on neuronal activity and proteotoxicity in C. elegans
Hirsch F
Dissertation 2025, Bremen, Germany - Convergent evolution of a fungal effector enabling phagosome membrane penetration
Jia LJ, Alexander Bernal F, Söhnlein J, Sonnberger J, Heineking I, Rafiq M, Cseresnyés Z, Kage F, Schmidt F, Zaher R, Hortschansky P, Chaudhary S, Gong X, Schirawski J, Thilo Figge M, Hube B, A.Brakhage A
bioRxiv 2025, https://doi.org/10.1101/2025.03. - Neuronal autophagy in the control of synapse function.
Karpova A, Hiesinger PR, Kuijpers M, Albrecht A, Kirstein J, Andres-Alonso M, Biermeier A, Eickholt BJ, Mikhaylova M, Maglione M, Montenegro-Venegas C, Sigrist SJ, Gundelfinger ED, Haucke V, Kreutz MR
Neuron 2025, 113(7), 974-90 - DNA damage response regulator ATR licenses PINK1-mediated mitophagy.
Marx* C, Qing* X, Gong* Y, Kirkpatrick J, Siniuk K, Beznoussenko GV, Kidiyoor GR, Kirtay M, Buder K, Koch P, Westermann M, Bruhn C, Brown EJ, Xu X, Foiani M, Wang ZQ
Nucleic Acids Res 2025, 53(5), gkaf178 * equal contribution - Replication stress responses in human lymphocytes change sex-specifically during aging.
Rall-Scharpf M, Schlotter D, Koch P, Szafranski K, Groth M, Sahm A, Biber S, Castaño BA, Heitmeir B, Zengerling F, Deniz M, Dallmeier D, Braig S, Bonig H, Milyavsky M, Pospiech H, Wiesmüller L
Nucleic Acids Res 2025, 53(11), gkaf498