Subarea 4: Cell Dynamics and Molecular Damages in Aging
The research focus of Subarea 4 is on studying damages of macromolecules (proteins, nucleic acids) and determining the structure-function relationship of biomolecules relevant to damage and damage repair processes and responses to molecular damage that might lead to aging and aging-associated pathologies.
The studies are focused on the following research areas: DNA replication, DNA damage responses (DDR), stress responses, metabolic stresses, protein trafficking and protein damages.
The research is defined by four focus areas:
- DNA damage response in tissue homeostasis and neuropathies,
- Quality control in the endoplasmic reticulum for secretory pathway in aging processes,
- Intrinsic and extrinsic factors implicated in cellular decline during aging, and
- DNA replication and genomic integrity preventing premature aging and diseases.
Research focus of Subarea 4.
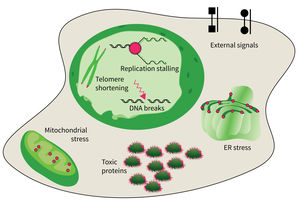
The accumulation of damaged macromolecules or subcellular organelles is associated with dysfunction of a cell, which contributes to tissue & organ failure. DNA damage, genomic instability, protein misfolding or defects in toxic protein degradation can compromise cell functionality. Alterations of mitochondrial DNA and protein complexes affect cellular metabolism, which will have a general impact on cell integrity.
Publications
(since 2016)
2024
- Amyloid beta precursor protein contributes to brain aging and learning decline in short-lived turquoise killifish (Nothobranchius furzeri)
E.M.de Bakker D, Mihaljević M, Gharat K, Richter Y, Bagnoli S, van Bebber F, Adam L, Shamim-Schulze F, Ohlenschläger O, Bens M, Cirri E, Antebi A, Matić I, Schneider A, Schmid B, Cellerino A, Kirstein J, Riccardo Valenzano D
bioRxiv 2024, https://doi.org/10.1101/2024.10. - Asparagine614 Determines the Transport and Function of the Murine Anti-Aging Protein Klotho.
Fanaei-Kahrani Z, Kaether C
Cells 2024, 13(20), 1743 - PARP1 UFMylation ensures the stability of stalled replication forks.
Gong Y, Wang Z, Zong W, Shi R, Sun W, Wang S, Peng B, Takeda S, Wang ZQ, Xu X
Proc Natl Acad Sci U S A 2024, 121(18), e2322520121 - HROB Is Implicated in DNA Replication.
Kutz J, Schmietendorf H, Rahman SA, Opel F, Pospiech H
Genes (Basel) 2024, 15(12), 1587 - Mutant huntingtin impairs neurodevelopment in human brain organoids through CHCHD2-mediated neurometabolic failure.
Lisowski P, Lickfett S, Rybak-Wolf A, Menacho C, Le S, Pentimalli TM, Notopoulou S, Dykstra W, Oehler D, López-Calcerrada S, Mlody B, Otto M, Wu H, Richter Y, Roth P, Anand R, Kulka LAM, Meierhofer D, Glazar P, Legnini I, Telugu NS, Hahn T, Neuendorf N, Miller DC, Böddrich A, Polzin A, Mayatepek E, Diecke S, Olzscha H, Kirstein J, Ugalde C, Petrakis S, Cambridge S, Rajewsky N, Kühn R, Wanker EE, Priller J, Metzger JJ, Prigione A
Nat Commun 2024, 15(1), 7027 - J-domain proteins: From molecular mechanisms to diseases.
Marszalek J, De Los Rios P, Cyr D, Mayer MP, Adupa V, Andréasson C, Blatch GL, Braun JEA, Brodsky JL, Bukau B, Chapple JP, Conz C, Dementin S, Genevaux P, Genest O, Goloubinoff P, Gestwicki J, Hammond CM, Hines JK, Ishikawa K, Joachimiak LA, Kirstein J, Liberek K, Mokranjac D, Nillegoda N, Ramos CHI, Rebeaud M, Ron D, Rospert S, Sahi C, Shalgi R, Tomiczek B, Ushioda R, Ustyantseva E, Ye Y, Zylicz M, Kampinga HH
Cell Stress Chaperones 2024, 29(1), 21-33 - Ultraviolet B acts as a dietary restriction mimetic by targeting mitochondrial bioenergetics
Martirosyan A, Li Y, Woitzat Y, Lee S, Li F, Ermolaeva MA
bioRxiv 2024, 10.1101/2024.03.05.583543 - HSP110 is a modulator of amyloid beta (Aβ) aggregation and proteotoxicity.
Montresor S, Pigazzini ML, Baskaran S, Sleiman M, Adhikari G, Basilicata L, Secker L, Jacob N, Ehlert Y, Kelkar A, Kalsi GK, Kulkarni N, Spellerberg P, Kirstein J
J Neurochem 2024, 169(1), e16214 - Compromised CDK12 activity causes dependency on the high activity of O-GlcNAc transferase.
Pallasaho S, Gondane A, Kutz J, Liang J, Yalala S, Duveau DY, Pospiech H, Thomas CJ, Loda M, Itkonen HM
Glycobiology 2024, 34(12), cwae081 - Functional study of SMG6-mediated mRNA decay in brain development
Pereira Carvalho Guerra G
Dissertation 2024, Jena, Germany