Jena/Tel Aviv. Just as countless letters or packages containing goods are sent in daily life, there are similar transport processes in every cell of our body. Every day, newly synthesized proteins, the "cargo," must be conveyed to their final destination with a high degree of reliability. Behind the scenes, this transport requires extensive logistics, because it is the only way that proteins can be selectively passed from station to station to be transported from their site of synthesis to other locations within the cell or out of the cell to several locations in the body.
One-third of all newly synthesized proteins in cells are routed via the so-called secretory pathway. This pathway is comprised of the endoplasmic reticulum (ER), the “factory”, and the Golgi apparatus, the main “logistics center” for further distribution of the goods in the cell. But how do the goods get from the factory to the logistics center, i.e. from the ER to the Golgi apparatus?
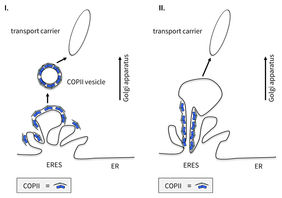
In cell biology, the prevalent view how cargo is transported from the ER to the Golgi is that small membrane-vesicles are loaded with the proteins to be transported and bud off the ER. They fuse to larger transport containers which are then transported along “railroad tracks”, the microtubules, to the Golgi. At the Golgi they fuse with membrane and release their cargo for further distribution. Important to this process is a special protein coat, the COPII complex, that helps in concentrating the cargo at the ER and budding off the small vesicles that are consequently called COPII-coated vesicles (transport vesicles; coat protein complex II). But this knowledge now needs to be revised.
Paradigm shift in cell biology
Israeli researchers led by Prof. Koret Hirschberg of Tel Aviv University (TAU) and German researchers led by Dr. Christoph Kaether of the Leibniz Institute on Aging - Fritz Lipmann Institute (FLI) in Jena, Germany, now suggest a different model for protein transport in the cell and the role of COPII in a study published in the Journal of Cell Biology.
"It was tough to get this paper published," says Prof. Hirschberg, "because it is against the dominating dogma in the field, the vesicle transport hypothesis. This study was possible only because of the extensive long term collaboration of the FLI and TAU teams."
COPII complex - First sorting station for proteins
Using high-end live-cell imaging combined with electron microscopy, the research team gained insights into living mammalian cells and was able to confirm through complementary biochemical analyses that the COPII components play an important role in the transport process of proteins. But not in the way that text books used to convey. Contrary to the prevalent view that COPII-enveloped vesicles transport proteins, COPII complexes do not leave the endoplasmic reticulum. They remain permanently at the exit sites of the ER, where their task is the recruitment, sorting, and exit of proteins from the ER.
COPII complexes are stable and immobile
Thus, proteins do not exit the ER in COPII-enveloped vesicles, as previously postulated, but in larger, often elongated COPII-free transport units. "Instead of budding off and enveloping vesicles, COPII acts like a gatekeeper right at the boundary between ER and ERES, the exit sites of the ER, where it controls and regulates the concentration of cargo," Dr. Kaether says.
At the same time a similar paper in Cell (https://doi.org/10.1016/j.cell.2021.03.035) by Jennifer Lippincott-Schwartz, a renowned membrane transport expert from the Howard Hughes Medical Institute, USA, came to identical conclusions using a set of different methods, strongly supporting the data from the FLI/TAU collaboration.
"Both papers change our current view on one of the most fundamental processes in cell biology, the export from the ER," declares Dr. Kaether, highlighting the findings. "Because this is such an essential pathway for the cell, it is not surprising that it plays an important role in the aging process and in many diseases - from common diseases such as cancer to neurological disorders and rare inherited diseases," adds Dr. Kaether, the co-corresponding author from FLI.
The data presented here lay the foundation for an alternative model, on the role of the COPII complex in export from the ER. A more detailed understanding of the underlying mechanisms of protein transport will help to better understand disease mechanisms and provide the basis for novel therapeutic approaches.
Publication
COPII collar defines the boundary between ER and ER exit site and does not coat cargo containers. Shomron O, Nevo-Yassaf I, Aviad T, Yaffe Y, Zahavi EE, Dukhovny A, Perlson E, Brodsky I, Yeheskel A, Pasmanik-Chor M, Mironov A, Beznoussenko GV, Mironov AA, Sklan EH, Patterson GH, Yonemura Y, Sannai M, Kaether C, Hirschberg K. J Cell Biol. 2021 Jun 7;220(6):e201907224. doi: 10.1083/jcb.201907224.
Contact
Dr. Kerstin Wagner
Press and Public Relations
Phone: 03641-656378, email: presse@~@leibniz-fli.de