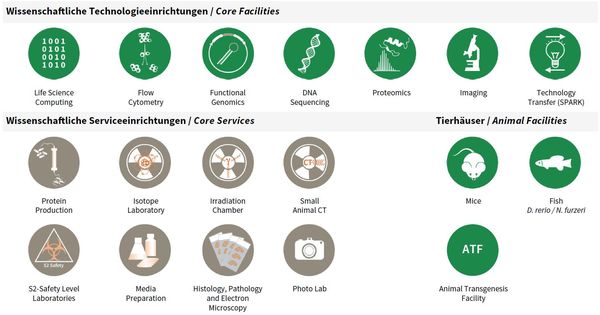
Core Facilites and Core Services
At the beginning of 2016, a “core” structure was put into effect that organized facility and service units as independent organizational entities from FLI’s research groups. A number of technology platforms (e.g. sequencing, mass spectrometry) grew out of individual methodological requirements for single research groups in the last years but developed into semiautonomous substructures. As consequence of re-focused research activities and the concomitant advent of new research groups at FLI, those units increasingly had to serve many FLI groups and collaborative research efforts in the Jena research area.
To accommodate this development and to increase efficiency as well as transparency for users, facility personnel and for administrative processes, it came natural to re-organize such activities into independent units as “FLI Core Facilities and Services” and to phase out infrastructures considered non-essential for FLI’s research focus (X-ray crystallography and NMR spectroscopy).
FLI’s Core Facilities (CF) are managed by a CF Manager and are each scientifically guided in their activities and development by an FLI Group Leader, as Scientific Supervisor. The animal facilities comprising fish, mouse and transgenesis are run separately, as they involve a more complex organizational structure. Basic Core Services (CS) are directly led by the Head of Core (HC), who in turn is supported by individual CS Managers.
All facilities and services, including animal facilities, have a valuable contribution to FLI’s research articles; e.g. from 2016–2018, to 54% of all peer reviewed research publications.
Overview Core Facilities and Core Services at FLI.
Publications
(since 2016)
2021
- COPII collar defines the boundary between ER and ER exit site and does not coat cargo containers
Shomron O, Nevo-Yassaf I, Aviad T, Yaffe Y, Erez Zahavi E, Dukhovny A, Perlson E, Brodsky I, Yeheskel A, Pasmanik-Chor M, Mironov A, Beznoussenko GV, Mironov AA, Sklan EH, Patterson GH, Yonemura Y, Sannai M, Kaether** C, Hirschberg** K
J Cell Biol 2021, 220(6), e201907224 ** co-corresponding authors - TRIP6 functions in brain ciliogenesis.
Shukla S, Haenold* R, Urbánek* P, Frappart L, Monajembashi S, Grigaravicius P, Nagel S, Min WK, Tapias A, Kassel O, Heuer H, Wang ZQ, Ploubidou** A, Herrlich** P
Nat Commun 2021, 12(1), 5887 * equal contribution, ** co-senior authors - HAT cofactor TRRAP modulates microtubule dynamics via SP1 signaling to prevent neurodegeneration.
Tapias* A, Lázaro* D, Yin* BK, Rasa SMM, Krepelova A, Kelmer Sacramento E, Grigaravicius P, Koch P, Kirkpatrick J, Ori A, Neri F, Wang ZQ
Elife 2021, 10, e61531 * equal contribution - Backbone and nearly complete side-chain chemical shift assignments reveal the human uncharacterized protein CXorf51A as intrinsically disordered.
Wiedemann C, Obika KB, Liebscher S, Jirschitzka J, Ohlenschlãger O, Bordusa F
Biomol NMR Assign 2021, 15(2), 441-8 - Backbone and nearly complete side-chain chemical shift assignments of the human death-associated protein 1 (DAP1).
Wiedemann C, Voigt J, Jirschitzka J, Häfner S, Ohlenschläger O, Bordusa F
Biomol NMR Assign 2021, 15(1), 91-7
2020
- Structural Requirements of the Phytoplasma Effector Protein SAP54 for Causing Homeotic Transformation of Floral Organs.
Aurin MB, Haupt M, Görlach M, Rümpler F, Theißen G
Mol Plant Microbe Interact 2020, 33(9), 1129-41 - Modulation of FLT3 signal transduction through cytoplasmic cysteine residues indicates the potential for redox regulation.
Böhmer A, Barz S, Schwab K, Kolbe U, Gabel A, Kirkpatrick J, Ohlenschläger O, Görlach M, Böhmer FD
Redox Biol 2020, 28, 101325 - Spatially resolved analysis of FFPE tissue proteomes by quantitative mass spectrometry.
Buczak K, Kirkpatrick JM, Truckenmueller F, Santinha D, Ferreira L, Roessler S, Singer S, Beck** M, Ori** A
Nat Protoc 2020, 15(9), 2956-79 ** co-corresponding authors - Structure of an RNA aptamer in complex with the fluorophore tetramethylrhodamine.
Duchardt-Ferner E, Juen M, Bourgeois B, Madl T, Kreutz C, Ohlenschläger O, Wöhnert J
Nucleic Acids Res 2020, 48(2), 949-61 - Loss of metabolic plasticity underlies metformin toxicity in aged Caenorhabditis elegans.
Espada* L, Dakhovnik* A, Chaudhari* P, Martirosyan A, Miek L, Poliezhaieva T, Schaub Y, Nair A, Döring N, Rahnis N, Werz O, Koeberle A, Kirkpatrick J, Ori A, Ermolaeva MA
Nat Metab 2020, 2(11), 1316-31 * equal contribution