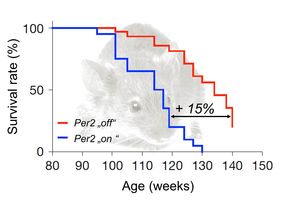
There’s no other age group suffering more from infectious diseases than seniors. With growing age, the risk of chronic and cute infections increases. This is due to the diminishing potential of hematopoietic stem cells (HSC) to build blood and immune cells in an appropriate number. In particular, HSC’s capability to build lymphocytes is strongly declining, which leads to imbalances in blood cell composition and, thus, to immune defects limiting overall fitness and organismal survival during aging. There is experimental evidence that the accumulation of DNA damage contributes to these aging-induced immune impairments. Now, a group of researchers lead by Karl Lenhard Rudolph, Scientific Director of Leibniz Institute on Aging – Fritz Lipmann Institute (FLI), identified gene “Per2” as a genetic switch for a better immune system in mice: Per2 gene deletion ameliorates DNA damage responses in HSC leading to stabilization of hematopoietic stem and progenitor cells in aging mice. Hence, mice were less prone to infections and exhibited an elongated lifespan by 15 % without increases in cancer. The results of the study are published online on April 18, 2016, in Journal Nature Cell Biology.
“Circadian Clock“-gene Per2 identified by a genetic screen
For their study, in vivo RNA-mediated interference (RNAi) screenings were conducted in mice. 459 putative tumor suppressor genes were targeted to identify genes that limit the self-renewal capacity of HSC in response to DNA damage and aging. This screen identified “period circadian clock 2 (Per2)”-gene – usually one out of various genes regulating sleep-wake cycle – to represent a major factor limiting the maintenance and repopulation capacity of HSC in the context of various types of DNA damage and aging. Interestingly, Per2 deletion was sufficient to maintain a balanced production of lymphocytes, and hence, to improved immune function in aging mice. A similar effect was also found for DNA damages caused by the shortening of telomeres – the protective caps at our chromosomes’ ends –, a mechanism though to be relevant for human aging.
A further step towards healthy aging
“All in all, these results are very promising, but equally surprising”, K. Lenhard Rudolph summarizes. “We did not expect such a strong connection between switching off a single gene and improving the immune system so clearly”. It will be of future interest to study if the results are transferable to humans. Although humans and mice are genetically quite similar, genes usually regulate myriad of processes in an organism, and possible side-effects of Per2 deletion will have to be elucidated very carefully. Interestingly, Per2 gene mutations in humans have been associated with advanced sleep disorders leading to advanced tiredness of the patients in the early evening hours. “It is not yet clear whether this mutation in humans would have a benefit such as improved immune functions in aging – it is of great interest for us to further investigate this” Rudolph says.
Publication
Wang J, Morita Y, Han B, Niemann S, Löer B, Rudolph KL. Per2 induction limits lymphoid-biased haematopoietic stem cells and lymphopoiesis in the context of DNA damage and ageing. Nature Cell Biology 2016 (e-pub ahead of print), DOI: 10.1038/ncb3342.
Contact
Dr. Evelyn Kästner
Leibniz Institute on Aging – Fritz Lipmann Institute (FLI)
Beutenbergstr. 11, D-07745 Jena
Tel.: + 49 3641-656373, E-Mail: presse@~@leibniz-fli.de